

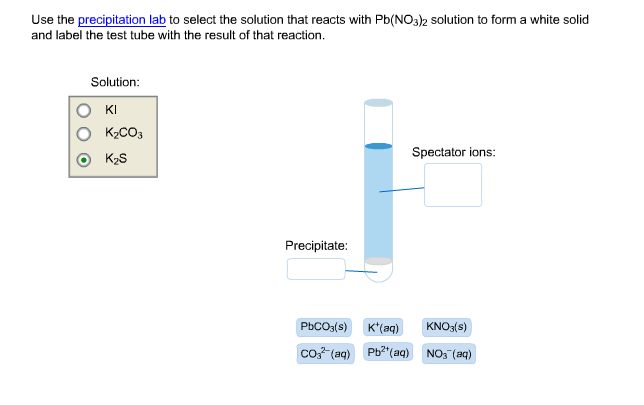
When we precipitate the harmful heavy metal ions, they are converted from the aqueous state (where they cause harm), to the solid state which can be trapped and filtered out of the solution.

Sometimes we need to use chemicals specifically designed to remove dirt. On the other hand, some dirt/stains build up slowly but they are harder to remove and can make workspaces harder to use.Many living organisms actually need these lighter ions for living processes. They are very soluble in water but are carried around quite easily too, and they do not build up to dangerous levels very often. In chemistry, light metal ions such as Na + or K + are fairly safe in living environments. Dust builds up but can be removed quickly too, so a general dry wipe across a surface is enough. Some dirt/stains are very light and don’t interfere with general working.Removing impurities or ‘treating’ a water sample is similar to cleaning a work space. Being able to precipitate ions out of solution is very important in chemistry.
#Pbco3 precipitate color how to
How to use K sp and ion concentrations to solve problems related to hard water and pollution.To recall the meaning of the terms suspension and supernatant.How precipitation is used to treat water pollution and hard water.1ġ Reference for solubility constant data: What amount of CO 3 2- ions need to be added to precipitate lead ions, so that drops to the acceptable level at most? The K sp for PbCO 3 is 7.4*10 -14 M. 1Ī sample of industrial waste water has a Pb 2+ concentration of 6.5*10 -3 M, and 'acceptable levels' of lead ions in water have been stated as a maximum of 7.25*10 -10 M. What amount of OH - ions need to be added to precipitate lead ions, so that drops to the acceptable level at most? The K sp for Pb(OH) 2 is 1.43*10 -20 M. Use the K sp expression to calculate concentrations of aqueous ions in treating water pollution.Ī sample of industrial waste water has a Pb 2+ concentration of 7.9*10 -4 M, and 'acceptable levels' of lead ions in water have been stated as a maximum of 7.25*10 -10 M.


 0 kommentar(er)
0 kommentar(er)
